By Nutifafa Adotey, Assistant Professor & Soil and Nutr. Magt Specialist, Forbes R Walker, Professor & Envt. Soil Specialist, and Frank Yin, Professor & Cropping System Scientist, University of Tennessee
In Tennessee, the primary N fertilizer sources used are urea and urea ammonium nitrate (UAN). Other sources of N fertilizers, such as anhydrous ammonia, are used in some counties in Central and West Tennessee. It is a known fact that ammonia volatilization from applied N fertilizers may occurs from (1) unincorporated, surface-applied urea-based fertilizer; (2) anhydrous ammonia due to poor equipment calibration; and (3) unincorporated, surface-applied ammonium-based fertilizer on alkaline soils. This N loss from these fertilizers is governed by a complex interaction of prevailing environmental conditions, soil properties and management practices. Soil properties determine the potential of ammonia loss while the extent of the loss is dictated by environmental conditions and management practices.
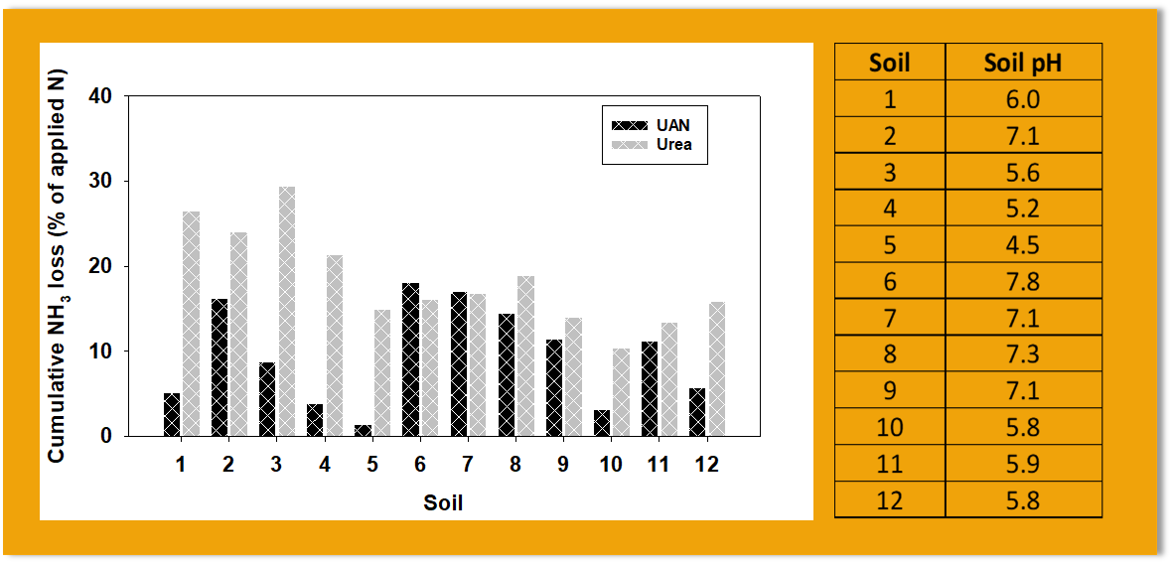
Normally, ammonia volatilization from surface application of UAN tends to be lower than surface application of urea. It’s important that farmers are well acquainted with situations when surface-broadcast of UAN results in higher ammonia loss than normal, and in some instances greater losses than urea. Soil pH and prevailing critical relative humidity are partly responsible for the higher than usual ammonia loss from UAN. In a lab study conducted at the WTREC on 12 soils with different soil pH (Funded by Tennessee Department of Agriculture). The cumulative ammonia volatilization losses 16 days after fertilizer application from UAN in soils 2, 6, 7, 8, 9, and 11 were higher than losses from UAN in the remaining soils (see figure above). Soil pH of each soil is shown at the right side of the graph. The pH of soils 2, 6, 7, 8, and 9 greater than 7.
Urea ammonium nitrate (UAN) is made up of urea, ammonium, and nitrate, in contrast to urea-N fertilizer which is composed of 100 % urea. Hence, the initial concentration of ammonium in UAN is high. In alkaline soils, the high soil pH favors the conversion of ammonium to ammonia . This explains why in soils with pH greater than 7, the ammonia loss from UAN is very high regardless of the buffering capacity of the soil. The initial pH plays a major role in ammonia loss from surface applied UAN. Also, the urea hydrolysis is limited in UAN so very little ammonia is formed from the urea portion of UAN compared to 100% urea. In contrast, the amount of ammonia loss from urea is dependent not only on the initial soil pH, but also on the buffering capacity of the soil. Applied urea undergoes urea hydrolysis to produce ammonium and bicarbonate, which results in an increased soil pH around the immediate environment of urea granules. In poorly buffered soils, the increase in soil pH may substantially drive ammonia volatilization from urea. However, in well buffered soil, the increase in pH may not drive ammonia loss from urea.
Field studies in (study 1 & study 2) Georgia have reported that UAN lost a similar or higher amount of ammonia than urea. The authors attributed the higher ammonia loss from UAN to the influence of critical relative humidity (CRH). The low CRH (18 – 20 %) of UAN than urea (72 – 80 %) meant that UAN absorbed moisture from its surroundings more rapidly than urea, which would only absorb moisture when its relative humidity is around 80 %.
Tennessee producers have multiple options to successfully minimize ammonia loss when applying UAN in fields with pH greater than 7. Surface-applied UAN should be treated with a proven nitrogen stabilizer for optimal yields when soils pH is above 7. Subsurface-banding and injection have been reported to reduce ammonia loss from UAN applied in fields with pH less than 7; however, there is limited information on the effect of these placement methods on ammonia loss when soil pH is greater than 7. Information on N stabilizers evaluated under growing conditions in Tennessee are available at https://utia.tennessee.edu/publications/wp-content/uploads/sites/269/2024/03/W1221.pdf and https://utia.tennessee.edu/publications/wp-content/uploads/sites/269/2023/10/PB1888.pdf.
Further Reading
Adotey, N., McClure, A.T., Yin, X., and J. McNeal. 2024. Performance of enhanced efficiency nitrogen fertilizers and sidedress nitrogen placement methods in dryland corn. UT Extension Publication, W1221.
Adotey, N., McClure, A.T., and X., Yin. Enhanced efficiency nitrogen fertilizer as a tool to control nitrogen loss in row crop production. UT Extension Publication, PB 1888
Cabrera, M.L., Franklin, D., Kissel, D.E., and J. Rema. 2023. Ammonia Volatilization from Urea and Urea-Ammonium Nitrate Solution Applied to a Grassland in the Southeastern USA. [Abstract]. ASA, CSSA, SSSA International Annual Meeting, St. Louis, MO. https://scisoc.confex.com/scisoc/2023am/meetingapp.cgi/Paper/153164
Vaio N., Cabrera M.L., Kissel D., Rema J.A., Newsome J.F., and Calvert V.H. 2008. Ammonia volatilization from urea‐based fertilizers applied to tall fescue pastures in Georgia, USA. Soil Science Society of America Journal 72:1665-1671. DOI: 10.2136/sssaj2007.0300.